Introduction to Innovative Nutraceutical Formulation
The development of new dietary supplement formulas is a complex and multidisciplinary process, involving a synergy between marketing, science, and regulation. In a dynamic market estimated at 1.9 billion euros in France, manufacturers must constantly innovate to meet the growing consumer demands for health and well-being, while navigating an increasingly strict regulatory environment.
Advantages of Powdered Dietary Supplements
Powdered dietary supplements offer several significant advantages over other dosage forms. Their high solubility facilitates rapid and effective absorption of nutrients by the body, thus maximizing their bioavailability. This form also allows for precise and customizable dosing, particularly useful for populations with specific nutritional needs, such as athletes, the elderly, and infants.
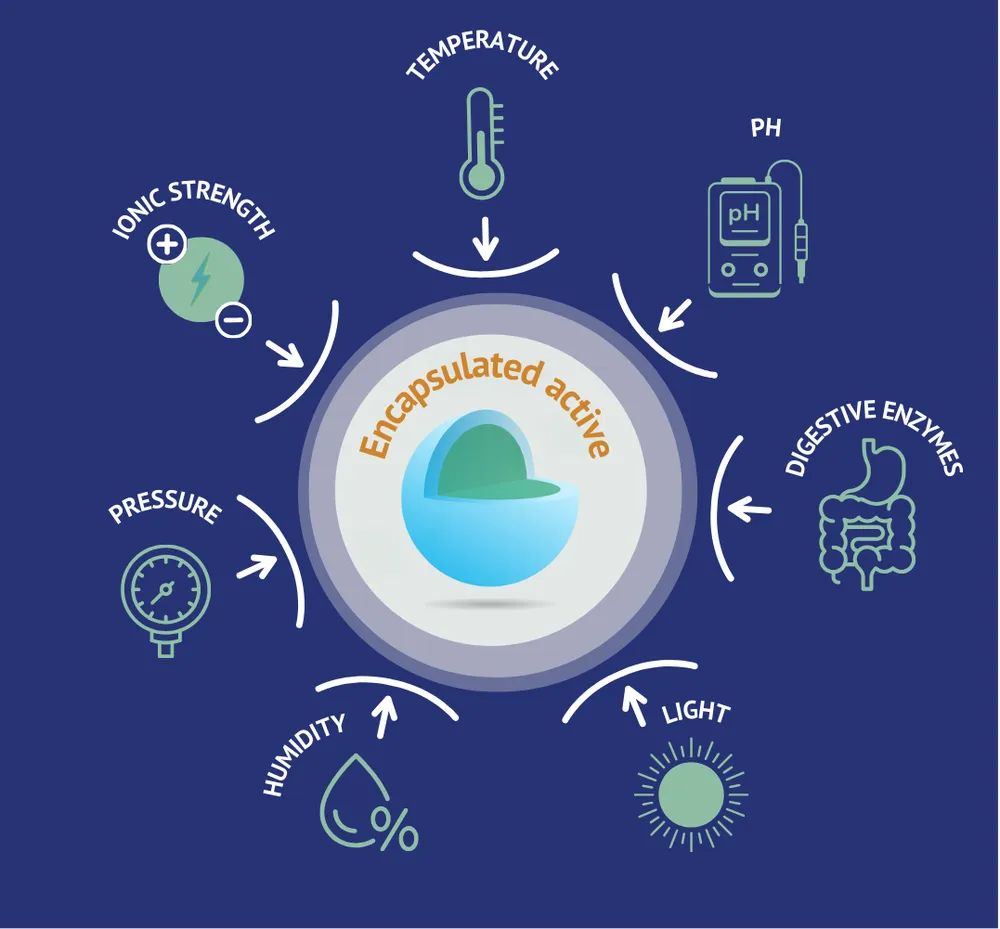
Moreover, powder offers flexibility in administration, easily incorporated into various drinks or foods, which improves treatment adherence. However, some compounds sensitive to oxidation or hydrolysis may be less stable in powder form, requiring advanced formulation techniques like encapsulation or the use of antioxidants to preserve their efficacy.
The Rise of Vegan Dietary Supplements
The rise of vegan dietary supplements reflects evolving dietary trends and the growth of the vegan market. With about 2% of the French population adopting a vegan diet in 2020, amounting to over 1.3 million people, the demand for nutritional supplements free from animal products has increased significantly.
The most crucial supplements for vegans include vitamin B12, essential for red blood cell formation and nervous system functioning, as well as iron, calcium, and vitamin D. Formulating these products presents unique challenges, particularly in terms of bioavailability and stability, requiring innovations in encapsulation and vectorization techniques to optimize the absorption of plant-based nutrients.
Regulatory Challenges of Dietary Supplements
The regulation of dietary supplements presents complex challenges for manufacturers, requiring constant vigilance and adaptation to legislative changes. Dietary supplements are subject to a strict regulatory framework, defined by the European Directive 2002/46/EC and the French Decree No. 2006-352, which classify them as foodstuffs intended to supplement the normal diet.
These products must comply with rigorous standards in terms of safety, composition, and labeling, including restrictions on nutritional and health claims governed by Regulation (EC) No. 1924/2006. A major challenge lies in the need to prove the efficacy and safety of ingredients, especially for new ingredients subject to Novel Food regulations. Additionally, managing adverse effects via the ANSES nutrivigilance system imposes increased post-marketing surveillance. Manufacturers must also navigate between sometimes divergent regulations of different countries, complicating international marketing and requiring sharp regulatory expertise to ensure the global compliance of products.

Nutraceutical R&D Process
Market Analysis and Commercial Strategy
Before embarking on formulation, it is crucial to conduct a thorough market analysis. This step involves:
- Competitor analysis (benchmarking) to identify existing products and opportunities for differentiation.
- Identifying consumer needs and defining a precise target audience.
- Evaluating market size, key players, distribution channels, and emerging trends.
This analysis allows for defining a strategic positioning and ensuring that the product meets a real market need.
Drafting the Specifications
The specifications document is key and defines the technical and economic specifications of the product. It should include:
- The expected benefits of the target product
- The physical and chemical characteristics/properties
- Regulatory constraints to comply with.
- Quality and safety criteria.
- Aspects related to packaging and product presentation.
Research and Development (R&D)
This crucial phase involves:
- Meticulous selection of ingredients based on scientific studies.
- Product formulation, requiring expertise in biology and chemistry to guarantee the supplement’s efficacy and stability.
Optimizing nutrient bioavailability, which may require advanced techniques like encapsulation or vectorization.
Ingredient Sourcing
Choosing suppliers is essential to ensure ingredient quality and traceability. This step includes:
- Selecting qualified suppliers (over 180 for some laboratories).
- Evaluating the quality, origin, and purity of ingredients.
- Considering ethical and environmental aspects (organic, ethical, traceability).
Prototyping and Testing
This phase involves:
- Producing pilot batches to verify industrial feasibility.
- Conducting stability tests to guarantee product preservation.
- Sensory analyses to evaluate organoleptic properties.
Regulatory Validation
Before market launch, it is imperative to ensure product compliance with current regulations:
- Verifying compliance with European Directive 2002/46/EC and French Decree No. 2006-352.
- Adhering to rules on nutritional and health claims (Regulation EC No. 1924/2006).
- Preparing notification dossiers for competent authorities.
Packaging and Labeling
This step includes:
- Choosing suitable packaging (capsules, tablets, powder, etc.).
- Designing labeling compliant with regulatory requirements, particularly in terms of nutritional information and claims.
Production and Quality Control
Manufacturing must follow Good Manufacturing Practices (GMP) and include:
- Implementing quality control procedures at each production stage.
- Conducting microbiological and physico-chemical tests on produced batches.
- Establishing a traceability system.
Marketing Strategy and Distribution
Finally, product launch requires:
- Developing a targeted marketing strategy.
- Choosing appropriate distribution channels (pharmacies, e-commerce, specialty stores).
- Setting up post-marketing monitoring to track any potential adverse effects.

Innov’ia Formulation Expertise
Formulation Expertise
Innov’ia possesses expertise in formulating functional ingredients, particularly suited to the infant nutrition and dietary supplement market. This expertise enables the development of innovative and effective formulations, meeting clients’ specific needs.
Advanced Technologies
The company has several industrial technological platforms, including:
- 13 fluidized bed units
- 8 spray drying towers, including 2 dedicated to prilling
These technologies enable the implementation of advanced processes such as dehydration, agglomeration, coating, and microencapsulation. These techniques are crucial for improving the stability, bioavailability, and efficacy of active ingredients in dietary supplements.
Microencapsulation
Innov’ia is a leader in microencapsulation, a technology particularly useful for protecting sensitive ingredients, controlling their release, and masking unpleasant tastes. This expertise is valuable for formulating more effective and palatable dietary supplements.
Research and Innovation
With over 30 years of know-how in formulation and a dedicated R&I center, Innov’ia can help develop innovative solutions to meet the specific challenges of each dietary supplement project.
Contract Manufacturing
Innov’ia offers tailor-made production capacities, enabling the transition from formulation to industrial-scale production. This flexibility is essential to meet clients’ varied needs, ensuring a smooth transition from development to commercialization.
Quality and Food Safety
Rigor in quality control and food safety is a priority at Innov’ia. Processes follow Good Manufacturing Practices (GMP) and include microbiological and physico-chemical tests at each production stage to guarantee product compliance and safety.
Certifications
2), Fami-QS, Kosher, Halal, and Organic. These certifications ensure that products meet the highest quality and safety standards, thus reinforcing the trust of clients and end consumers.
Multi-Market Expertise
Innov’ia’s experience in various markets, including human and animal nutrition, infant nutrition, pharmaceuticals, and cosmetics, allows for a transversal and innovative approach in the design of dietary supplements. This versatility is a major asset for developing solutions adapted to different industrial sectors.
Development of Specific Products
Innov’ia has expertise in formulating specific ingredients such as:
- Ingredients to strengthen immune defenses
- Foods for special medical purposes
- Prebiotics and probiotics
- Thickening agents
- Dietary supplements
This ability to develop targeted and effective products makes Innov’ia a key partner for companies seeking to innovate in the field of nutritional ingredients.

Conclusion
In conclusion, the formulation of dietary supplements is a multidisciplinary process requiring expertise in marketing, science, and regulation. The services offered by Innov’ia, ranging from formulation to industrial production, including advanced technologies like microencapsulation, position the company as an essential partner for businesses aiming to develop innovative, effective products that comply with the strictest quality standards.
FAQ
Spray drying is a dehydration method that disperses a liquid into fine droplets in a stream of hot air to obtain a dry powder.
They offer rapid absorption, precise dosing, flexible administration, and improved nutrient bioavailability.
Innov’ia provides formulation expertise, advanced technologies, contract manufacturing capabilities, and quality certifications to develop innovative and effective dietary supplements.
Innov’IA is certified FSSC 22000, GMP (part 2), Fami-QS, Kosher, Halal, and Organic.
Innov’ia combines technical expertise, advanced technologies, flexible production capacities, and commitments to quality and safety, making it a partner of choice for companies wishing to innovate in the field of dietary supplements.
Contact-us
"*" indicates required fields